Stop drowning in regulatory updates
We track FDA, MDCG, EU Official Journal, and more official sources so you don't have to. When something changes, you get the redline, the summary, and what to do next.
THE PROBLEM
Regulatory monitoring shouldn't take you days
The information is scattered.
You piece it together from ChatGPT searches, LinkedIn posts, newsletters, and colleague tips. You're never sure you haven't missed something.
Cutting through the noise takes forever.
When you do subscribe to official sources, you're buried in updates, and most don't apply to you. Finding what matters takes hours.
Understanding changes takes even longer.
Once you find a relevant update, you need to figure out what exactly changed. Two documents side by side, line by line comparison, to determine what's different and if it affects your products.
And then, you still must act on the update.
Once you have assessed the impact of an update, the real work begins. You have to run your gap analysis, update your documentation, train other teams… the list goes on.
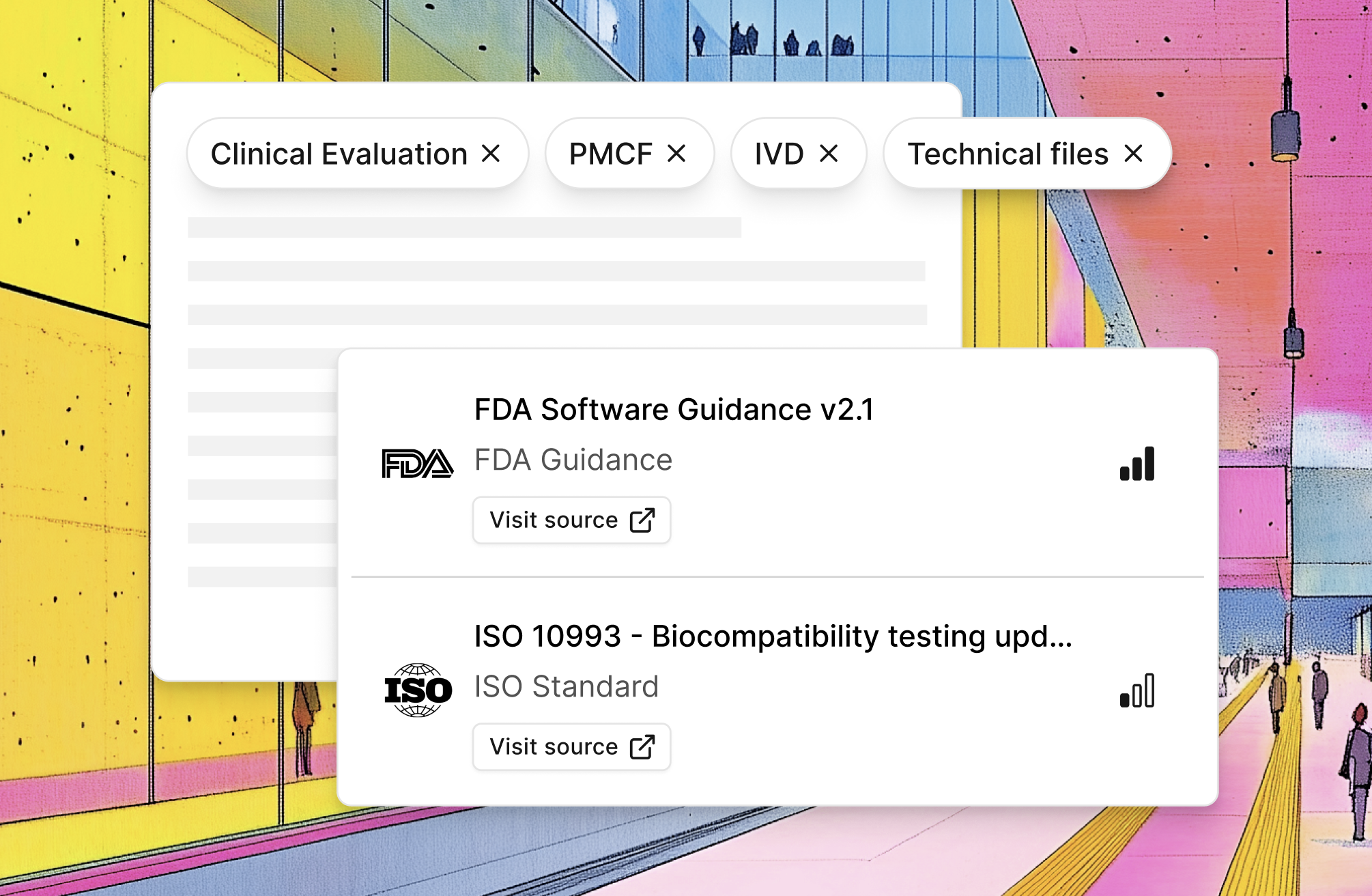
Filtered monitoring you can trust
Filtered by your topics — Clinical Evaluation, PMCF, IVD, Technical Files. Not everything. What you work on.
All official sources — FDA, EU Official Journal, MDCG, ISO, IMDRF, national authorities. Monitored systematically.
Always linked — Direct links to official documents. No hallucinations. Verify in one click.

Impact assessment, already done
Redlining built in — See exactly what changed between versions. Additions, deletions, modifications—all highlighted.
Impact assessment — Not just "something changed" but "here's whether it affects you and how urgently."
Action recommendations — Clear next steps for your compliance work and QMS documentation.
Comprehensive source monitoring for complete coverage
We monitor official EU, US, and international regulatory sources so you don't have to — regularly updated and verified by QARA experts.






European Commission Announcements, FDA Guidance Documents, MDCG and MEDDEV Guidance, US Federal Register, ISO/IEC Standards, EU NANDO Database, IMDRF Guidance, National Authorities (ANSM/BfArM), and more...
Ready to stay compliant without the overwhelm?
Join QARA professionals getting personalized, regulatory intelligence every month.